2) This #tweetorial, accredited for #CME/CE and intended for #physicians #physicianassociates #nurses #nursepractitioners #pharmacists is supported by an educational grant from Esperion Therapeutics. See faculty disclosures at https://t.co/gvXca4G9Xm.
— cardio-met (@cardiomet_CE) April 5, 2022
4) #ASCVD results from the retention of #apolipoprotein B containing lipoproteins mostly in the form of low-density lipoproteins (#LDL) in the vessel wall. LDL particles carry the majority of cholesterol in the circulation.
— cardio-met (@cardiomet_CE) April 5, 2022
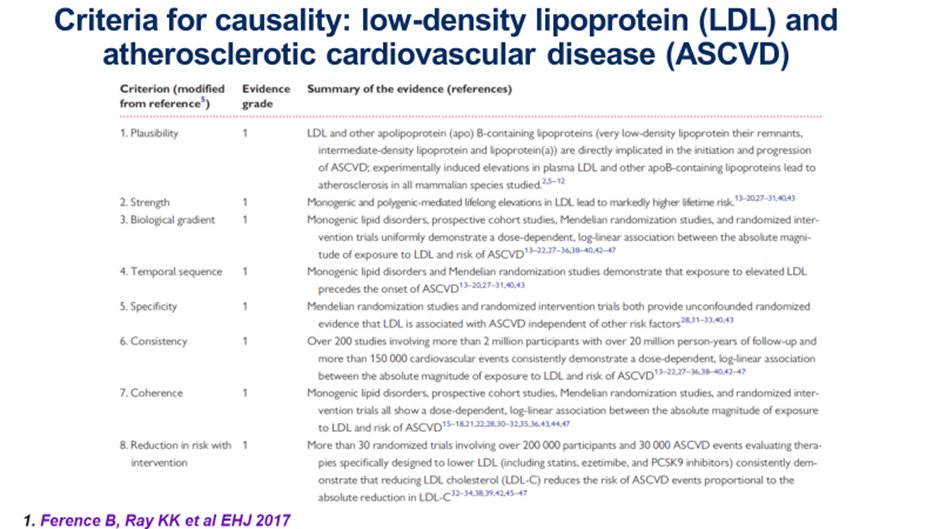
6) LDL-C fulfills the Bradford Hill Criteria for #causality, namely biological plausibility, biological gradient, strength of association, temporal relationship, specificity for ASCVD, consistency of the totality of data, external & internal validity & 🔑: reversibility. pic.twitter.com/jZCh4fNswv
— cardio-met (@cardiomet_CE) April 5, 2022
8) Apo-B containing lipoproteins up to 70 nm in diameter can cross the endothelium. As LDL is the most abundant lipoprotein in the circulation it is the key deliverer of cholesterol to the artery wall.
— cardio-met (@cardiomet_CE) April 5, 2022
9) Many risk factors modulate the propensity of LDL-C to traverse the endothelium and enter the arterial intima. See 🔓https://academic.oup.com/eurheartj/article/41/24/2313/5735221.
10) It now appears that the passage of #LDL into the #intima is not a merely passive process whereby the concentration in blood & the permeability of the endothelium determine LDL accumulation.

11) It’s #Transcytosis (an active process), through a vesicular pathway involving #caveolae, scavenger receptors (#SRB1) and activin like receptor kinase 1 (#ALK1). Hence for a given blood level of LDL-C the amount of atherosclerosis is variable.
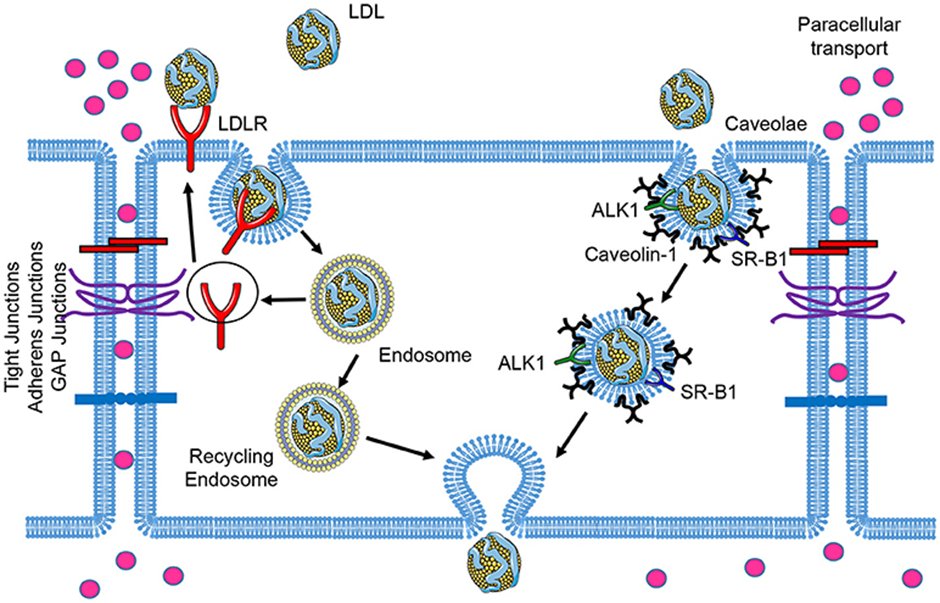
12) Both quality and quality of the apoB containing lipoproteins can contribute to the clinical #ASCVD phenotype. Again, see 🔓https://academic.oup.com/eurheartj/article/41/24/2313/5735221.
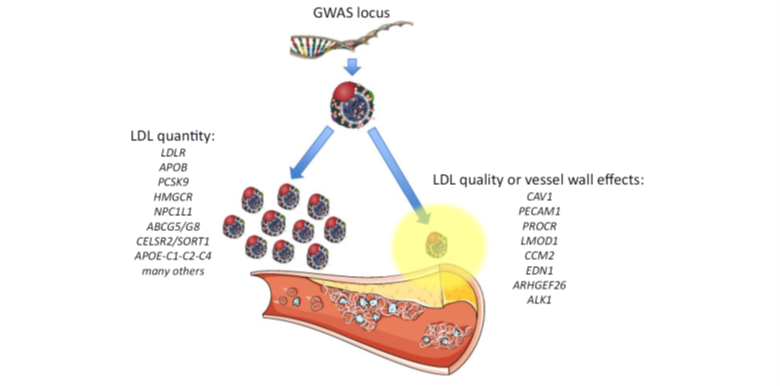
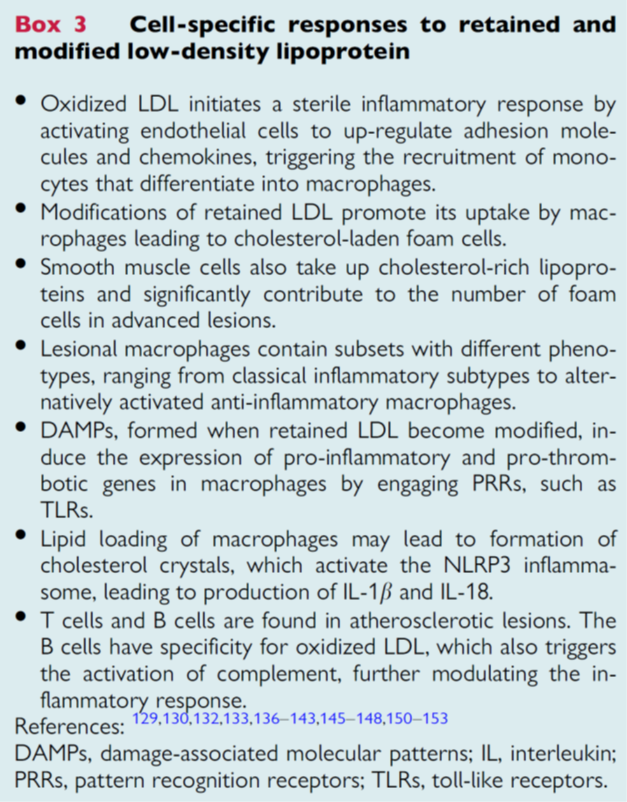
13) Cell-specific responses to retained and modified #LDL include a sterile inflammatory response through expression of adhesion molecules and attracting monocytes which differentiate into #macrophages.
14) Retained #LDL particles are taken up by macrophages resulting in #foamcell formation. Lipid retention in macrophages can lead to #NLRP #inflammasome activation. 🔓
15) Large-scale genetic studies show that #CV outcomes not only depend upon duration of exposure to #LDL-C, but are agnostic to the method by which it is achieved. A unit difference in LDL-C achieved . . .
16) . . . through genetic variations in pathways that mimic #statins (#HMGCoA), #ezetimibe (#NCP1L1), #PCSK9 (monoclonals or #inclisiran), ACLY (bempedoic acid), results in similar differences in risk of #CHD. See 🔓
17) Similarly in #RCTs of statins and #PCSK9i when #LDL-C differences are standardized per unit change and duration of treatment, similar relative risk reductions are observed.
18) This suggests that although statins are associated with changes in inflammatory markers as well as #LDL-C, and #PCSK9i with reductions in LDL-C and Lp(a), virtually all of the clinical benefit of these treatments at a population level are explained by changes in LDL-C.
19) So, a quick knowledge ✔️–were you paying attention? The process by which LDL-C enters arterial intima is known as:
20) Mark your response and return TOMORROW for more education on the inescapable links between #LDL-C & #atherosclerosis! Shout-outs to @GoggleDocs @Drlipid @SABOURETCardio @DrMarthaGulati @pnatarajanmd @mmamas1973 @DLBHATTMD @maciejbanach @drpablocorral @pabeda1 @TomPMarshall
21a) Welcome back! I am @ProfKausikRay & you are earning CE/#CME while we talk about #LDL-C➡️#atherosclerosis➡️⬆️#CV risk. 👏to @TomLuscher @fikkumamoto @natraff8 @ShivaniM_KC @mvaduganathan @KoushikReddyMD @drpratikc @kaulcsmc @AnumSaeedMD @PamelaBMorris @fjpinto1960
21b) Yesterday's quiz? Scroll back up to tweet 19 and answer if you didn't already. It's TRANSCYTOSIS–an active process by which #LDL-C enters the intima.

22) So, to the primary prevention setting. Alas, data from #WOSCOPS suggest that for those with LDL-C > 190mg/dl, risk calculators underestimate 10 year risk!
23) For instance 2/3 of patients with LDL-C > 190mg/dl would be predicted to have 10y risk below 7.5% and thus not offered statins. Yet their observed risk was twice that. This group derived greater absolute benefits.
24) In the same study long-term extension data from #WOSCOPS, over a further 15 years beyond the initial 5, suggested greater relative benefits over time (cumulative exposure), accruing, reducing #CHD deaths, #CV deaths and all-cause #mortality.
25) Clinical trials of LDL-C lowering have been harmonised using individual participant data on about 200,000 participants per 1mmol/L or 38mg/dl show that LDL-C lowering with statins . . .
26) . . ⬇️risk in relative terms similarly in those, w/ & w/o CVD, w/ & w/o risk factors but absolute benefit per unit change depends upon baseline risk, with those at greatest risk of MACE deriving greater absolute benefits. See 🔓https://www.thelancet.com/journals/lancet/article/PIIS0140-6736%2812%2960367-5/fulltext
27) As increasingly guidelines recommend lower cholesterol targets for those at highest risk, this inevitably will require greater use of combination therapies.

28) For patients requiring > 50% LDL-C lowering to get to goal, or unable to tolerate statin regimens which could provide 50% lowering, due consideration to combination should occur early. 🔓
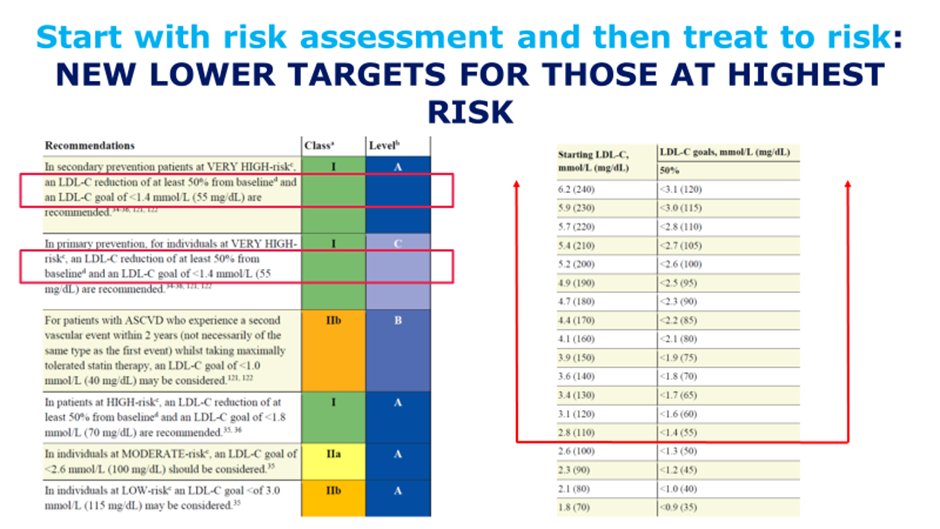
29) Combination therapies such as statins + #ezetimibe should be the norm for those patients at highest risk for whom lower LDL-C goals are recommended, thereby reducing unnecessary steps and clinical inertia.
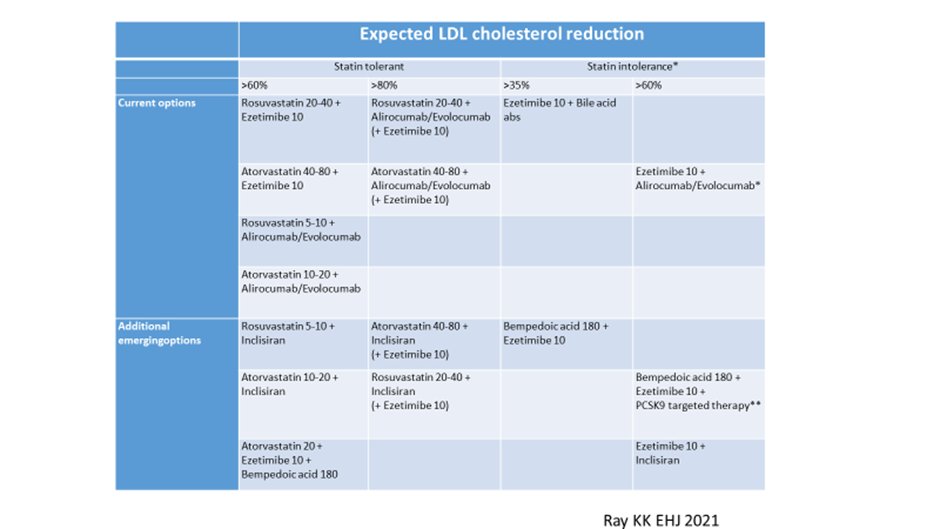
30) If more add-on therapy is needed, options include a third oral agent such as #bempedoic acid, or injectable therapies directed against #PCSK9.
31) For those who are statin intolerant, combination therapy with statins, bempedoic acid, & ezetimibe should be first line oral options, with the addition of injectable therapies as needed.
32) Time can be your friend: by starting #LDL-C lowering early, to derive greater cumulative benefits. Benefits from small reductions started early=large reductions required later.
33) Achieve the LDL-C that is right for your patient, i.e. match degree of LDL-C lowering to risk.
34) Those who have highest risk should achieve lower LDL-C levels to mitigate that risk. Combination therapies are likely needed when aiming for lower LDL-C. Several synergistic combinations are available both as oral therapies and injectable agents.
35) For those at highest RISK , get LDL-C low and keep it low!
36) So, one last knowledge✔️. Were you keeping up? Early consideration of #combination lipid-lowering therapy #LLT should be initiated when statin monotherapy does not ➡️what % desired #LDL-C lowering?
37) Did you answer before scrolling here? As per Tweet 28, for patients requiring > 50% LDL-C lowering to get to goal, or unable to tolerate statin regimens which could provide 50% lowering, due consideration to combination should occur early. Now go forth and treat!
38) And that's it! You made it! I am @ProfKausikRay and I hope you learned useful info that will impact your practice. Now you can grab 0.5h CE/#CME for 🆓at https://cardiometabolic-ce.com/lipids4/. Please FOLLOW US for more education from the #cardiometabolic experts!
Originally tweeted by cardio-met (@cardiomet_CE) on April 5, 2022.